The FDA has issued a recall for a boutique hand sanitizer brand due to potential harmful side effects, including blindness and coma.
The recall covers 40 batches of Aruba Aloe Balm, Aruba Aloe Hand Sanitizer Gel Alcohol 80%, and Aruba Aloe Alcoholada Gel.
The presence of methanol, a toxic substance found in vehicle antifreeze liquid, was detected in the contaminated hand sanitizer batches.
While typical side effects of methanol poisoning include vomiting, nausea, and headaches, more severe side effects such as coma, blindness, seizures, and even death are also listed among potential risks.
Aruba Aloe Balm N.V. Issues Voluntary Nationwide Recall of Aruba Aloe Hand Sanitizer Gel Alcohol 80% and Aruba Aloe Alcoholada Gel Due to Presence of Methanol https://t.co/QgqraeEXyr pic.twitter.com/nfEvzBOWGY
— U.S. FDA Recalls (@FDArecalls) April 8, 2024
According to the CDC, “Methyl alcohol (CH3OH) is a colorless liquid with a strong odor. It is a poisonous substance that can be absorbed through the eyes, skin, lungs, and digestive system. Overexposure can cause death. Workers may be harmed by exposure to methyl alcohol. The level of harm depends upon the dose, duration, and work being done.”
CBS News reported:
The recalled products were distributed between May 1, 2021, and Oct. 27, 2023, and sold in the U.S. online through the Aruba Aloe Balm website.
The company is notifying consumers who purchased the products by email and offering a discount coupon for a next purchase.
Those who purchased the recalled products should discard them.
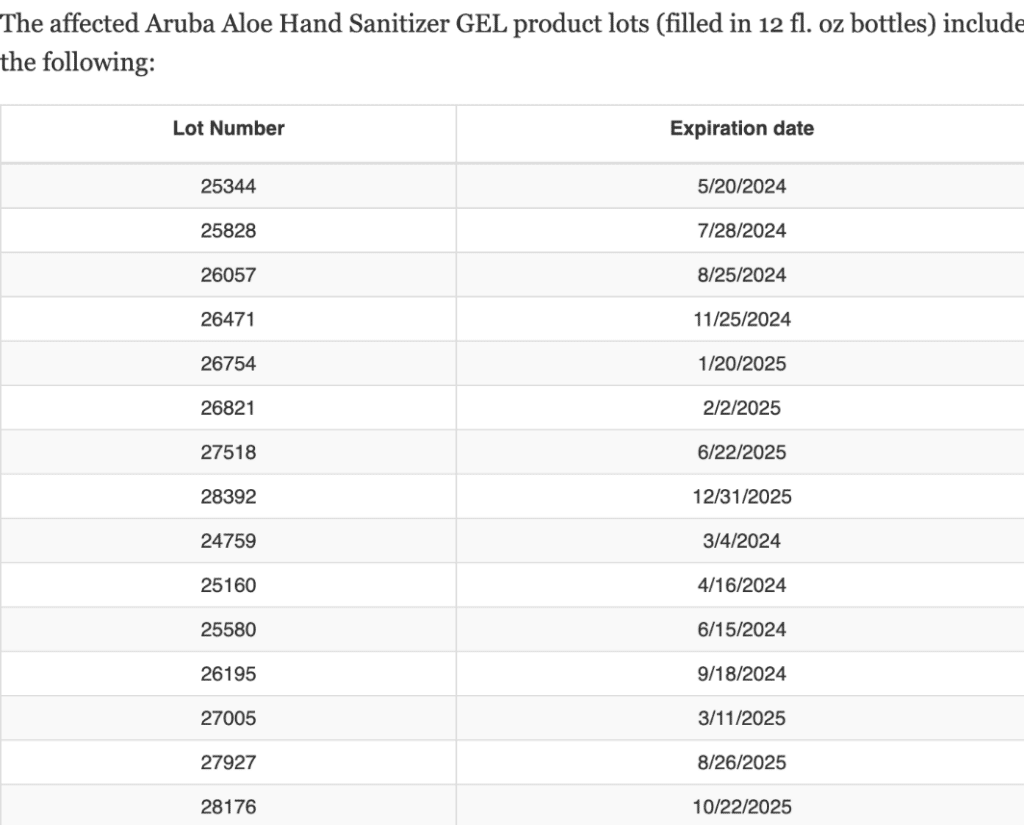
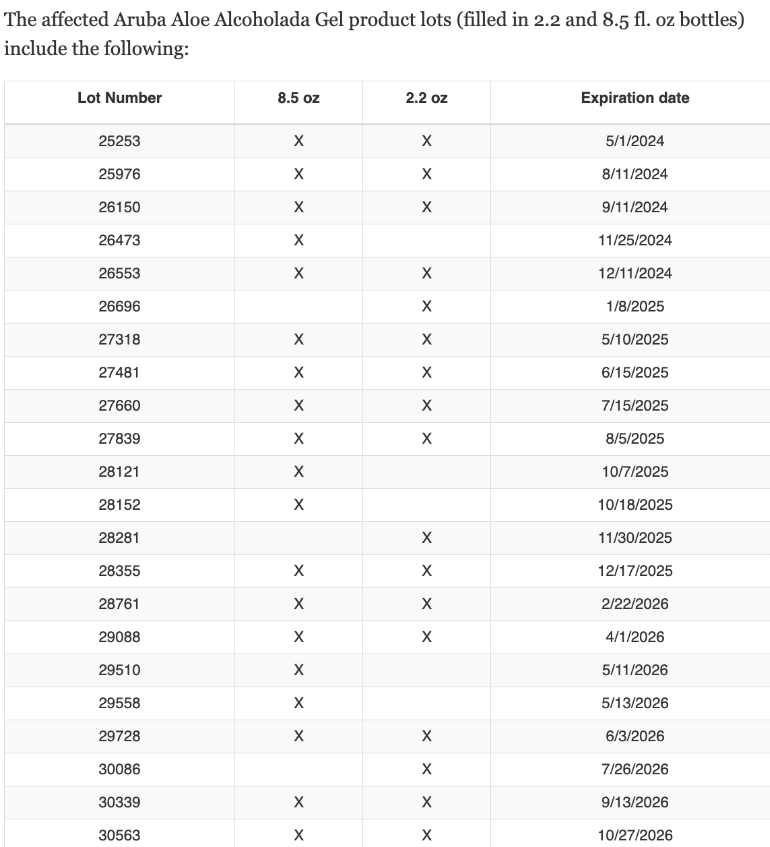
The Food and Drug Administration provided the following tables detailing the affected product batches, organized by lot number and expiration date.