
According to data the CDC turned over under court order, the earliest demographics to get COVID-19 injections, such as healthcare workers, reported a high rate of serious complications.
“Among the 10 million-plus users of the agency’s v-safe active monitoring smartphone app through July — 8.5 million of whom signed up between December 2020 and April 2021, before all adults were eligible for COVID vaccines — nearly 8% said they required medical care after receiving the vaccines,” Just the News reports.
From Just the News:
For patients ages 3 and older needing such care, nearly 3 in 4 couldn’t rely on telehealth visits. They required urgent care (48%), emergency room (15%) or hospitalization (10%). For infants, who were authorized to receive the jabs this summer and are enrolled in v-safe through parents or guardians, hospitalizations were much lower (2%) but urgent care much higher (66%).
Another 12% of v-safe users reported they were unable to perform normal daily activities, and 13% said they missed work or school, meaning 1 in 3 had more than mild adverse reactions.
Well over 10 million symptom reports were filed each month from January through April 2021, dropping to 5 million in May and hovering around 1 million for the next few months. The reports jumped above 2 million again in October following President Biden’s vaccine mandates for roughly 100 million workers, and dropped to the low- to mid-hundred thousands from January through July 2022.
The v-safe data obtained thus far are posted by the Informed Consent Action Network as both interactive graphs and several gigabytes of files. It got them through ongoing Freedom of Information Act litigation against the CDC.
Chest pain and other cardiac symptoms that could indicate myocarditis and pericarditis are completely missing from the survey checkboxes.
Aaron Siri wrote in Injecting Freedom:
As you can see, v-safe only collected certain limited, pre-selected information in a systematic fashion. For the first seven days after a shot, it asked users to check one or more of the following symptoms:
• chills
• headache
• joint pain
• muscle or body aches
• fatigue or tiredness
• nausea
• vomiting
• diarrhea
• abdominal pain
• rashDuring these first seven days, and then once a week for six weeks, and then at six months and one year, it asked users to pick, if applicable, one or more of the following three “health impacts:”
• unable to perform normal daily activities
• missed work/school
• needed medical careFinally, if a user selected that he or she needed medical care, v-safe would ask the user to select one or more of these options:
• hospitalization
• emergency room
• urgent care
• telehealthThat is most of the safety information, other than the free text fields, that v-safe collected.
Precisely what v-safe data did the CDC produce?
So, precisely what v-safe health data did the CDC produce to ICAN? The data produced to date consists of responses to the check-the-box fields in the screenshots above. It is worth reviewing the relevant screenshots again so you can see the health information that was gathered and provided to ICAN, keeping in mind that only the responses to the check-the box questions (not the free-text questions) have been provided thus far. It is important to understand what was (and what was not) captured by v-safe as we continue to break down the v-safe saga. How did the CDC decide to ask for only the above information?A simple review of the information requested in the health check-ins may leave you wondering why some obvious symptoms and adverse events you would expect v-safe to collect are not being collected – like chest pain or any other cardiac symptoms. You may ask how the CDC determined what to ask v-safe users. And that is a great question. First, let’s remind ourselves what was known about potential adverse events before any Covid-19 vaccine was administered to the general public:
- A July 2020 New England Journal of Medicine study titled “An mRNA Vaccine against SARS-Cov-2 – Preliminary Report” highlighted 35 adverse events that were related to the mRNA vaccination, including eye disorders, gastrointestinal disorders, musculoskeletal and connective tissue disorders, and nervous system disorders.
- An October 16, 2020 JAMA article titled “Postapproval Vaccine Safety Surveillance for COVID-19 Vaccines in the US” stated that “AESIs [Adverse Events of Special Interest] are likely to include allergic, inflammatory, and immune-mediated reactions, such as anaphylaxis, Guillain-Barré syndrome, transverse myelitis, myocarditis/pericarditis, vaccine-associated enhanced respiratory disease, and multisystem inflammatory syndrome in children.”
- In a CDC presentation dated October 30, 2020, titled “CDC post-authorization/post-licensure safety monitoring of COVID-19 vaccines,” a preliminary “list of VSD pre-specified outcomes for RCA [rapid cycle analysis]” and “list of VAERS AEs[ adverse events] of special interest” both included acute myocardial infarction, anaphylaxis, convulsions/seizures, encephalitis, Guillain-Barre syndrome, immune thrombocytopenia, MIS-C, myocarditis/pericarditis, and transverse myelitis, among others.
Again, the fact that mRNA can cause these serious conditions was raised before the first Covid-19 vaccine was authorized for use by the general public in December 2020 – in fact, months before.
Reflecting the concern that mRNA vaccines can cause these serious conditions, the CDC’s own protocol for v-safe, at least as early as January 28, 2021 (we are, on behalf of ICAN, working to get earlier versions), identified “Adverse Events of Special Interest” which it placed in a chart entitled “Prespecified Medial Conditions.” This included 15 serious conditions of special interest to track after Covid vaccination.
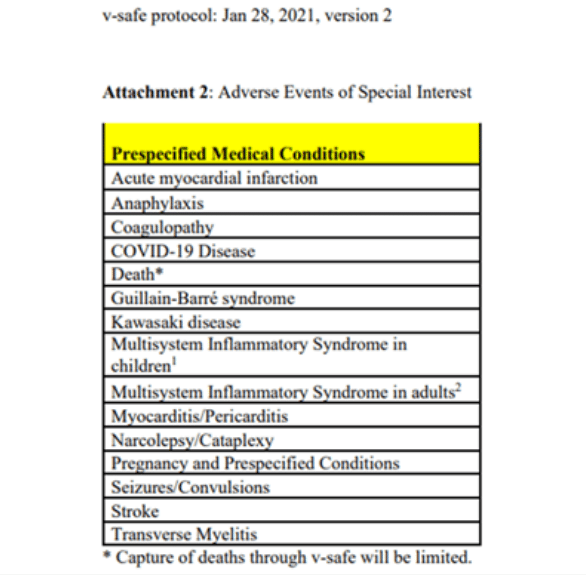
Again, this list was from the CDC’s own protocol used to develop v-safe. And, as seen above, this list included, among other serious concerns, myocarditis, pericarditis, acute myocardial infarction, stroke, GBS, and transverse myelitis. Yet v-safe was launched without including any check-the-box fields for these conditions and v-safe was never subsequently updated to include any check-the-box fields for these conditions.



